This unit was about three different subjects: the properties of water, the 4 main macromolecules, and enzymes. We went over the basics: the periodic table, atoms, and elements. Then, we learned about three different bonds: ionic (atoms giving up electrons), covalent (atoms sharing electrons), and hydrogen (only applies to water, positive regions are attracted to negative regions). Also, we learned about different properties of water. Polarity is the unequal distribution of charges between H and O. Cohesion is when water bonds with water, adhesion is when water bonds with something else, and capillary action is when the force of the H-bonds are stronger than the force of gravity.

Next, we learned about carbohydrates, which are made of sugar rings and provide energy. Lipids are fats, phosolipids, oils, etc. and they store energy. Proteins are structural and are enzymes, and they provide support to the body. Nucleic acids are made up nucleotides stranded together, and they are a source of information. Finally, enzymes are proteins that speed up chemical reaction by lowering the activation energy needed. They can be affected by pH and temperature and can become denatured, which means they won't work. I also learned more about sugars, factors that affect enzymes, and enzymes themselves.
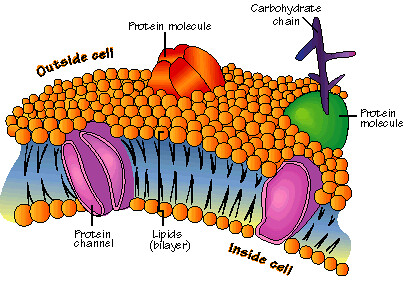
I had success in understanding most of the content that was represented to me. I did all of the vodcasts, and what I didn't understand was answered in class. Most of the things I learned in this unit were things I already knew about, but I got more information and explanations about them. This unit was good for expanding on what I already knew before. One setback I had was the part of the labs, because I think our group could have made some errors on steps of the procedures. I think that when we do labs, we can all follow the procedures better, and try not to make errors that would affect our results. I am better from what I was at the beginning of the unit because now I know more and will do better work now that I understand how everything works.
I want to learn more about some of the macromolecules. What we learned about the 4 different molecules did not really talk about how different molecules affect the macromolecules. Also, I want to know how it all works together in one system. Hopefully as we go on in this school year, I will understand and know more about this subject.
The purpose of this lab was to find how the structure of a carbohydrate changes how it tastes. So the question for this lab was, "How does the structure of a carbohydrate affect its taste (sweetness)?" So, my hypothesis was, "If fructose is tasted, it will be very sweet because it is a monosaccharide. On my data table, which was filled out after the taste test, has the sweetness level of fructose at 150, which was 50 points higher than the second-most sweet substance, which was sucrose at 100. In fact, the sweetness level of monosaccharides were higher than that of disaccharides and polysaccharides. The polysaccharides were the least sweet of the group and were rated at an average of 20 on the sweetness scale. Disaccharides however, were much sweeter, but still on the low side, at an average of 65. As the saccharides gained more rings they became less and less sweeter. It's possible that more rings on the sugar cause the sugar to be less sweet. The sugars, as they got more complex, got less sweet. All of this evidence supports my claim because monosaccharides are the sweetest out of all the different sugars tested in this experiment. All of the other sugars were not as sweet and some, like cellulose and starch, were not sweet at all, and tasted more bitter. All in all, fructose was the sweetest because it is a monosaccharide.
The saccharides with less rings are more likely to be used for energy. The polysaccharides are used for structure in organisms and can also store energy. The disaccharides in the middle of the group can be in both food for energy and structure in the body of an organism. The monosaccharides are produced as the result of photosynthesis. Different saccharides with different structures serve different purposes.
Not all testers put the same rating for each sugar. One reason for that could be that people didn't discuss and agree on one rating. Also, a mistake could have been made in placing the sugars on the paper. For example, two sugars could have mixed. Lastly, all people taste things differently, so one person wouldn't taste something the exact same way another person did. According to the U.S. National Library of Medicine, taste can be determined by a variety of different factors (texture, temperature, smell, etc.). Of course, these factors will not be the same for every single taster in this lab. Also, different peoples' taste bud sense different things and send different signals to the brain. This is why one taster could rank the sweetness of different sugars differently than others.
In this lab, we asked the question, "What concentration of bleach is best to fade the color out of new denim material in 10 minutes without visible damage to the fabric?" We found that a 100% concentration of bleach fades the jeans the most, but it causes some damage to the jeans themselves. The 50% concentration of bleach solution faded the denim best without causing much damage to the jeans. In a data table used to collect information about the lab, the color and damage to the denim was ranked from one to ten. The damage to jeans from O% concentration, 12.5% concentration, and 25% concentration was ranked at 0. The average color change for the 50% denim was 5, and the damage was 2. However, for the 100% jeans, the color change was 8, but the damage was ranked at 3. The jeans that were soaked into 100% bleach were whitish and yellow, while the 50% solution denim were light blue. This data supports our claim because the 50% jeans had the least damage while fading the jeans to a light blue color. The 100% jeans were damaged and a yellowish color. Therefore, the jeans soaked with the 50% solution were faded the best without much visible damage.
While our hypothesis was supported by our data, there could have been errors due to the fact that the jeans were washed for a slightly longer time than the procedure said. This in turn could have made all the colors of the jeans more blue than they were supposed to be. Our data contradicts the expected results because we rounded our averages to the nearest whole number. This may have made the data less precise than it would have been with decimals in them. Due to these errors, in future experiments I would recommend to start and stop timers and parts of the experiment more accurately, and I would also make the data averages more precise by rounding to the nearest tenth or hundredth of a number.
This lab was done to demonstrate our understanding of the scientific method and our ability to use it with reliability. From this lab I learned how to actively use the scientific method and not just know about it. This will help me understand the concept of doing labs properly, safely, and in the correct way. Now I can use the method whenever I do any other lab in this class. Based on my experience from this lab, I will do labs related to biology in the right way while following a procedure, collecting data, and writing a conclusion based on the scientific method.




